Atlanta, GA…On May 18, 2022, a U.S. resident tested positive for monkeypox after returning to the U.S. from Canada. As of May 18, 2022, no additional monkeypox cases have been identified in the U.S. Scientists at the Centers for Disease Control and Prevention (CDC) are collaborating with the Massachusetts Department of Public Health to investigate a situation in which a U.S. resident tested positive for monkeypox on May 18 after returning to the U.S. from Canada.
CDC is also tracking multiple clusters of monkeypox that have been reported in early- to mid-May in several countries that don’t normally report monkeypox, including in Europe and North America.
For more information on exposure risk, see Monitoring Persons Exposed.
It’s not clear how people in those clusters were exposed to monkeypox but cases include people who self-identify as men who have sex with men. CDC is urging healthcare providers in the U.S. to be alert for patients who have rash illnesses consistent with monkeypox, regardless of whether they have travel or specific risk factors for monkeypox and regardless of gender or sexual orientation.
What people should do:
People who may have symptoms of monkeypox should contact their healthcare provider. This includes anyone who:
traveled to central or west African countries, parts of Europe where monkeypox cases have been reported, or other areas with confirmed cases of monkeypox during the month before their symptoms began,
reports contact with a person with confirmed or suspected monkeypox, or
is a man who regularly has close or intimate contact with other men, including through an online website, digital application (“app”), or at a bar or party.
Recommendations for Clinicians
If clinicians identify patients with a rash that could be consistent with monkeypox, especially those with a recent travel history to central or west African countries, parts of Europe where monkeypox has been reported, or other areas reporting monkeypox cases, monkeypox should be considered as a possible diagnosis.
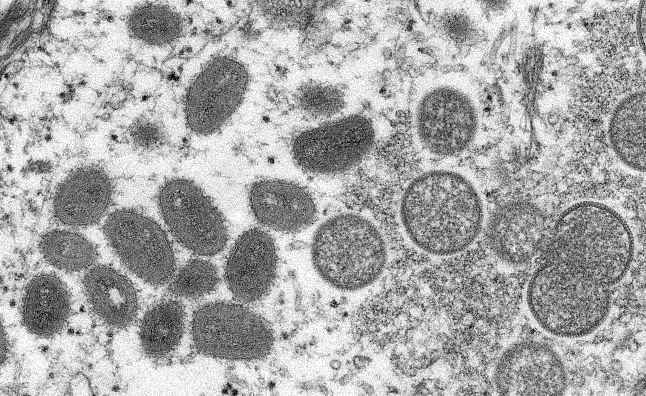
The rash associated with monkeypox involves vesicles or pustules that are deep-seated, firm or hard, and well-circumscribed; the lesions may umbilicate or become confluent and progress over time to scabs.
Presenting symptoms typically include fever, chills, the distinctive rash, or new lymphadenopathy; however, onset of perianal or genital lesions in the absence of subjective fever has been reported.
The rash associated with monkeypox can be confused with other diseases that are encountered in clinical practice (e.g., secondary syphilis, herpes, chancroid, and varicella zoster). However, a high index of suspicion for monkeypox is warranted when evaluating people with a characteristic rash, particularly for men who report sexual contact with other men and who present with lesions in the genital/perianal area or for individuals reporting a significant travel history in the month before illness onset or contact with a suspected or confirmed case of monkeypox.
Information on infection prevention and control in healthcare settings is provided on the CDC website Infection Control: Hospital | Monkeypox | Poxvirus | CDC. CDC is currently reviewing this information to consider the need for updates.
Clinicians should first consult their state health department (State Contactsexternal icon) or CDC through the CDC Emergency Operations Center (770-488-7100) as soon as monkeypox is suspected.
All specimens should be sent through the state/territorial public health department, unless authorized to send them directly to CDC.
Recommendations for Health Departments
If monkeypox is suspected, CDC should be consulted through the CDC Emergency Operations Center (770-488-7100).
Appropriately collected samples can be sent to CDC or an appropriate Laboratory Response Network (LRN) laboratory for testing by PCR.
Laboratory Response Network laboratories are able to provide orthopoxvirus testing on lesion specimens that clinicians obtain from suspected patients; confirmatory monkeypox virus-specific testing at CDC requires a dry lesion swab specimen.
Vigorously swab or brush lesion with two separate sterile dry polyester or Dacron swabs;Collect multiple specimens for preliminary and confirmatory testing as follows:
Break off end of applicator of each swab into a 1.5- or 2-mL screw-capped tube with O-ring or place each entire swab in a separate sterile container. Do not add or store in viral or universal transport media.